Terminology in Thermodynamics
Terminology in Thermodynamics: Overview
This topic covers concepts, such as, Thermodynamics, Scope of Thermodynamics, Path Function & Cyclic Process etc.
Important Questions on Terminology in Thermodynamics
Which of the following are not state functions?
During isothermal expansion of an ideal gas, its
For a cyclic process, which of the following is not true?
At constant temperature, for a given mass of an ideal gas
The total number of intensive properties from the following is _____.
Volume, Molar heat capacity, molarity, ,Gibbs free energy change, Molar mass, Mole
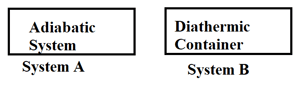
What happens when methane undergoes combustion in systems A and B respectively?
Number of intensive properties are:
, Molarity, Gibbs free energy, Molar mass, Mole, Molar heat capacity?
Is work done intensive or extensive property?
The container that suits the occurence of an isothermal process should made of
Establish the adiabatic equation constant.
Select the false statement from the following.
The diagram shows the pressure and volume relationship for one cycle of operation of an engine.
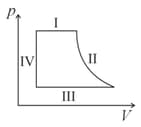
Which of the labelled parts of the cycle identify isobaric changes and adiabatic changes of state?
Which of the following option is correct match for extensive and intensive property:
Which of the following statements is true for an open system?
Work done by an ideal gas at a constant volume is__________
Which of the following is an extensive property?
For a thermodynamic system, which among the following is not a state function?
Which of the following variables control the physical properties and behaviour of the gas?
When heat is added to a gas constantly, it results in
An isolated system is that system in which:
